It’s back to the future for the generally discarded nickel-iron battery, a rechargeable technology developed by Thomas Edison more than a century ago.
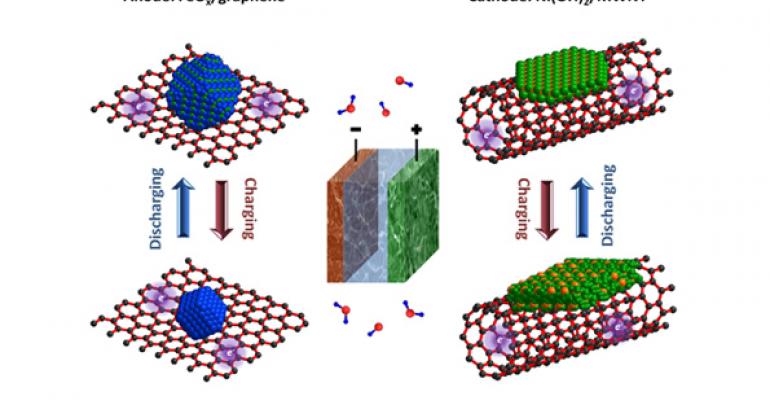
Stanford University scientists have developed an ultrafast Edison battery by growing iron oxide crystals on graphene sheets and nickel hydroxide crystals on multi-walled carbon nanotubes.
Designed in the early 1900s to power electric vehicles, the Edison battery largely went out of favor in the mid-1970s. Only a handful of companies manufacture nickel-iron batteries, primarily to store surplus electricity from solar panels and wind turbines.
“The Edison battery is very durable, but it has a number of drawbacks,” Stanford chemistry professor Hongjie Dai says in a statement. “A typical battery can take hours to charge, and the rate of discharge is also very slow.”
But this is about to change.
Dai and colleagues report in the journal Nature Communications they have created an ultrafast nickel-iron battery that can be fully charged in about two minutes and discharged in less than 30 seconds.
“We have increased the charging and discharging rate by nearly 1,000 times,” says graduate student Hailiang Wang, lead author of the study. “We've made it really fast.”
The high-performance, low-cost battery some day could be used to help power electric vehicles, much as Edison originally intended, Dai says. “Hopefully we can give the nickel-iron battery a new life.”
Edison, an advocate of all-electric vehicles, began marketing the nickel-iron battery around 1900. It was used in electric vehicles until about 1920. The battery's long life and reliability made it a popular backup power source for railroads, mines and other industries until the mid-20th century.
Edison created the nickel-iron battery as an inexpensive alternative to corrosive lead-acid batteries. Its basic design consists of two electrodes, a cathode made of nickel and an anode made of iron, bathed in an alkaline solution.
“Importantly, both nickel and iron are abundant elements on Earth and relatively nontoxic,” Dai says.
To improve the Edison battery's performance, the Stanford team uses graphene – nano-sized sheets of carbon only one atom thick – and multi-walled carbon nanotubes, each consisting of about 10 concentric graphene sheets rolled together.
“In conventional electrodes, people randomly mix iron and nickel materials with conductive carbon,” Wang says. “We grew nanocrystals of iron oxide onto graphene, and nanocrystals of nickel hydroxide onto carbon nanotubes.”
This technique produced strong chemical bonding between the metal particles and the carbon nanomaterials, which had a dramatic effect on performance.
“Coupling the nickel and iron particles to the carbon substrate allows electrical charges to move quickly between the electrodes and the outside circuit,” Dai says. “The result is an ultrafast version of the nickel-iron battery that's capable of charging and discharging in seconds.”
At this stage, the 1V prototype battery developed in Dai's lab has just enough power to operate a flashlight. The researchers are working to make a bigger battery that could be used for the electrical grid or transportation.
Most electric cars, such as the Nissan Leaf and Chevrolet Volt, run on lithium-ion batteries, which can store a lot of energy but typically take hours to charge.
“Our battery probably won't be able to power an electric car by itself, because the energy density is not ideal,” Wang says. “But it could assist lithium-ion batteries by giving them a real power boost for faster acceleration and regenerative braking.”
The prototype battery has one key drawback: the ability to hold a charge over time.
“It doesn't have the charge-discharge cycling stability that we would like,” Dai says. “Right now, it decays by about 20% over 800 cycles.” That's about the same as a Li-ion battery.
“But our battery is really fast, so we'd be using it more often. Ideally, we don't want it to decay at all.”
“The use of strongly coupled nanomaterials represents a very exciting approach to making electrodes,” he adds. “It's different from traditional methods, where you simply mix materials together. I think Thomas Edison would be happy to see this progress.”